Jonathan King, Professor of Molecular Biology, MIT
Melissa Kosinski-Collins, Professor of Biology, Brandeis University
Eric Sundberg, Professor of Biochemistry, Emory University School of Medicine
Structure and Organization of Coronaviruses
Many concerned over the coronavirus outbreak may find it useful to understand more about coronaviruses than is currently being communicated by media sources. As long-time structural biologists we offer below basic information on coronavirus, that may be of assistance to those who have not studied virology.
All viruses are parasites which can only reproduce within cells. Thus, they are very different from bacteria and fungi, which are self-reproducing, often in soil, water, organic wastes, sewage, or within organisms.
Animal and plant viruses fall into two general classes, those in which the genetic material is long DNA molecules, and those in which the genetic material is RNA molecules. Among the DNA viruses are Herpes, Adenoviruses, and wart viruses. Coronaviruses, named for their “sun-like” shape observed in the electron microscope, use RNA molecules to encode their genes, as do influenza viruses, HIV, and rhinoviruses (common cold). SARS-CoV-2, the virus that causes COVID-19, infects mammals and birds. It is closely related to the viruses causing the earlier SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) outbreaks.
The coronavirus particles are organized with long RNA polymers tightly packed into the center of the particle, and surrounded by a protective capsid, which is a lattice of repeated protein molecules referred to as coat or capsid proteins. In coronavirus, these proteins are called nucleocapsid (N). The coronavirus core particle is further surrounded by an outer membrane envelope made of lipids (fats) with proteins inserted. These membranes derive from the cells in which the virus was last assembled but are modified to contain specific viral proteins, including the spike (S), membrane (M), and envelope (E) proteins.
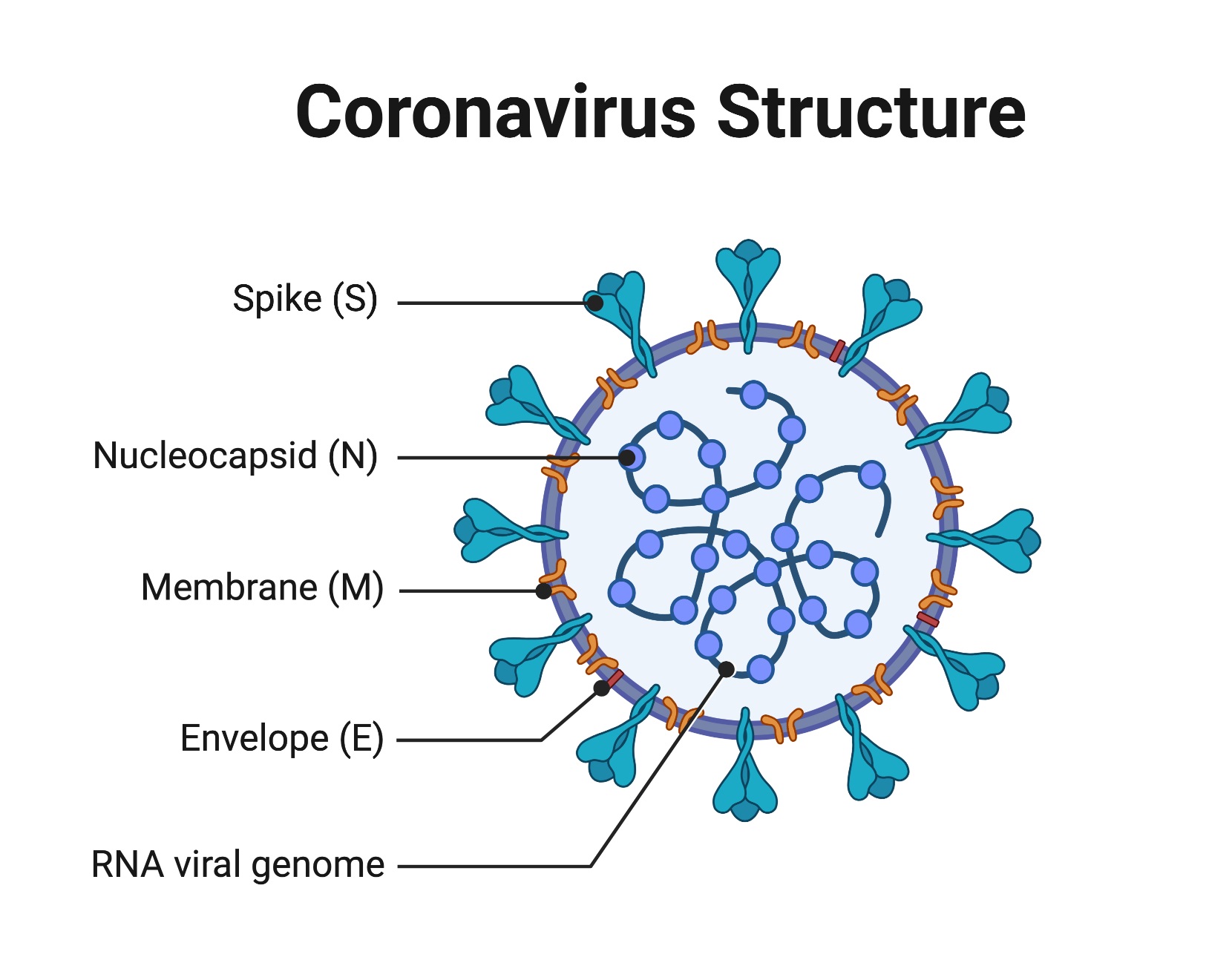
A key set of the proteins in the outer membrane project out from the particle and are known as spike proteins (S). It is these proteins which are recognized by receptor proteins on the host cells which will be infected.
Coronavirus particles are rapidly inactivated – killed – by exposure to 70% ethanol or 90% isopropanol (rubbing alcohol), hydrogen peroxide solutions, hypochlorite bleach, soaps and detergents, as well as by UV light and the high temperatures of cooking.
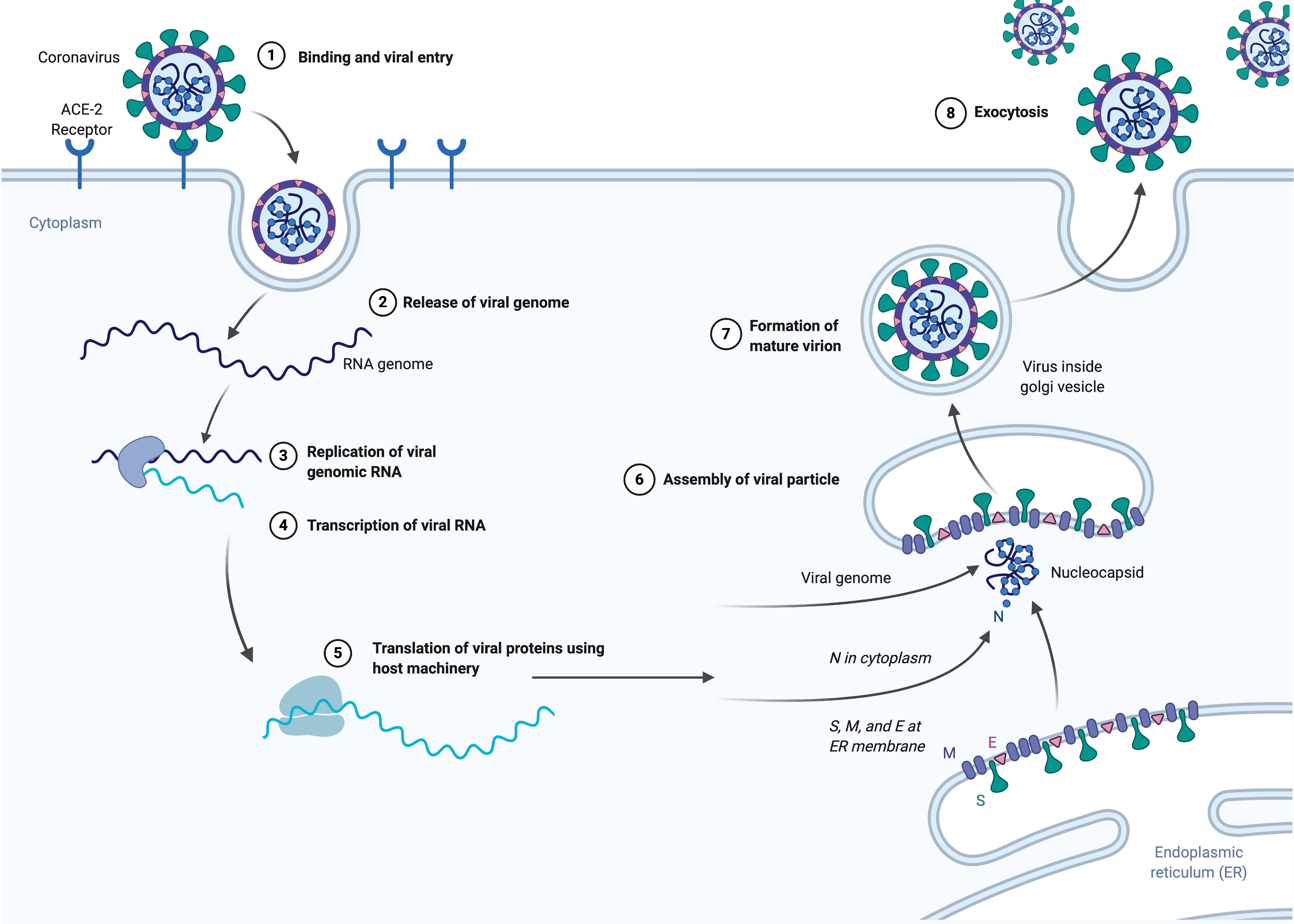
Coronaviruses primarily infect human lung cells through a receptor for an enzyme called Angiotensin Converting Enzyme 2 (ACE2). ACE2 is a member of the family of angiotensin converting enzymes that includes ACE, for which many Americans take blood pressure medicines composed of chemicals that act by inhibiting ACE. As the first step leading to viral infection, the virus spike protein recognizes and binds to the ACE2 receptor. The virus is then incorporated into the lung cells and the viral RNA is released into the cytoplasm. The viral RNA molecules recruit the cellular apparatus to make thousands of copies of the viral RNA and also instruct the cells to synthesize hundreds of thousands of nucleocapsid, membrane, envelope, and spike proteins. These assemble into new virus particles which bud out of the cell surface membrane. The cells release the newly formed viral particles propagating the infection and eventually die.
Testing for the Virus
The nucleotide sequence of the viral RNA molecules is not found in human DNA or RNA sequences. The test for the presence of the virus, thus, tests for the presence of the viral RNA sequences in tissue samples. The current assay technology is called “RT-PCR.” RT stands for Reverse Transcriptase, an enzyme which copies RNA sequences into DNA sequences. PCR stands for Polymerase Chain Reaction, which reproduces and amplifies the DNA sequences for subsequent breakdown for determining the order of the individual nucleotides strung together in the original RNA polymer. The kits also require short DNA sequences called primers, which are synthesized in the laboratory.
The existence of these assays is testimony to the value of prior investment of federal National Institutes of Health, National Science Foundation, and Department of Energy funds into genomics and sequencing technology. The test requires adequate supplies of two enzymes and the primers, specialized instruments for running the reaction at elevated temperatures, and trained personnel. Hundreds of colleges and universities across the nation provide the training needed, but not the actual employees conducting the tests. Ramping up capacity to be able to perform millions of tests requires billions of dollars in immediate investment.
A more traditional test for virus infection is the presence of antibodies (more below) that bind to the virus. Such tests identify individuals who are now healthy but have previously been infected. Antibody tests require a small drop of blood and are much more rapid than the current nucleotide sequencing tests. The absence and/or poor implementation of both RT-PCR and antibody-based tests early in the outbreak represents one of the failures of our healthcare/public health system to properly prepare for viral outbreak, particularly after the experiences of the SARS and MERS viruses.
The Immune System and Vaccine Development
Our blood, lymph, and organs are host to the white cells of the immune system, which are continually checking for the presence of foreign elements such as viruses, bacteria, fungi, parasites, tumor cells, and toxins. These white cells are made in the bone marrow.
One type of white cell is called a B-cell. B-cells are specific to a particular pathogen and secrete antibodies that detect that pathogen. Antibodies are proteins that bind to a foreign antigen, inactivate it, and/or target it for destruction by other white cells. When a person is infected by a foreign substance, B-cells will begin making and excreting antibodies into the bloodstream that recognize the outer surface of the pathogen. For viruses, it is often the spike proteins that are recognized. Some antibodies that bind to the viral spike proteins can prevent the viral particles from infecting the cells. Other white cells (macrophages) can engulf these compromised particles, removing them from circulation. B-cells can also keep a “memory” of the antibody that recognized a past pathogen and, in the case of another exposure, mount a response that is quicker and more efficient.
A second class of white cells are known as “killer” cells. Some of these white cells can recognize a cell that is infected by the virus and kill those cells. The phlegm that you cough up in a respiratory infection is full of debris from infected cells lysed by killer white cells.
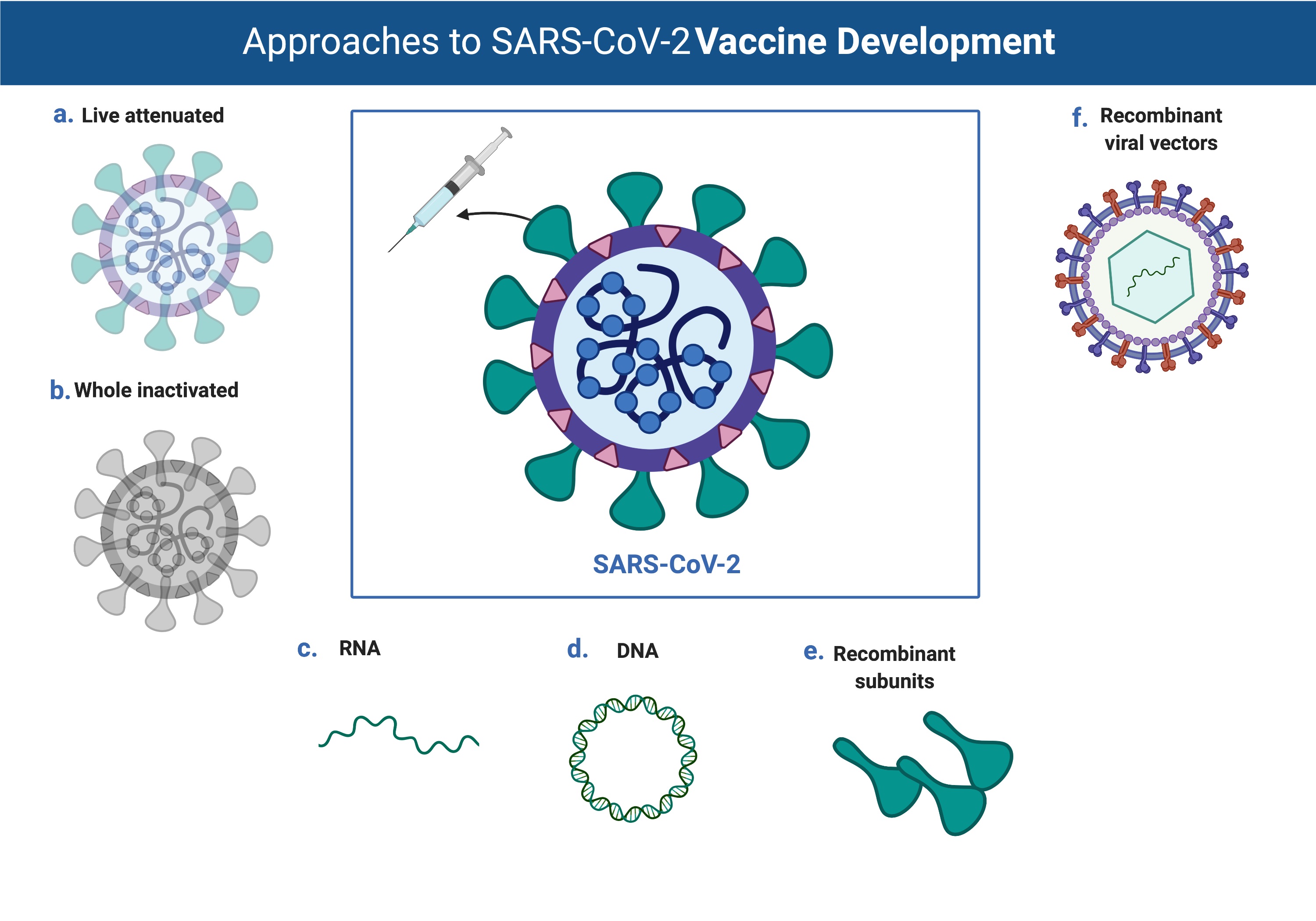
One of the best ways to protect against infection is to stimulate the immune system with a vaccine. For example, the polio vaccine consists of inactivated viral particles. These are unable to initiate an infection but are recognized by the white cells of the immune system. Over a period of weeks, the white cells that recognize the virus reproduce in the body. These white cells synthesize and secrete antibodies that can bind to the virus in the vaccine. If the individual is then exposed to infectious poliovirus, the circulating antibodies are already present and are able to inactivate the infecting particles. This immunity may last for decades, though that differs depending on the antigen.
Developing a vaccine requires growing large amounts of virus, often in animals, or in tissue culture at large scale. The viruses are inactivated by radiation, heat, or chemicals, or are derived from genetically weakened strains. Another alternative is to purify not the complete virus, but isolated viral proteins like spike. This is safer and easier to scale up, but the immune system response to the isolated protein is often not as robust as it is to the organized lattice of the intact virus particle. A more recent strategy involves injecting individuals with RNA or DNA encoding for viral proteins. These nucleic acids can be administered alone or through man-made vectors that help deliver material into the body. In any strategy, however, enough material is needed to inject reasonable doses into millions of people.
But before doing this one has to know that the vaccine works to stimulate a protective immune response. This requires recruiting human volunteers to be vaccinated and then be challenged with the infectious virus. All of this takes time and skilled personnel and money. However, with sufficient investment, success is highly likely in most cases. Note that vaccination is typically preventive – most vaccines do not provide relief for someone already infected.
Antiviral Therapies for Infected Individuals
Addressing the health hazards of coronavirus infections would benefit greatly by anti-viral drugs that act to block the attachment and internalization process or the replication of the virus within infected cells. Antivirals that interfere with the viral life-cycle without significantly impacting normal cellular function are critical to combating viral infections. Such therapies are in use for other RNA viruses, like influenza, and are administered generally as small molecules, taken in pill form. These antivirals act by binding to and interfering with viral proteins needed to replicate the viral RNA or facilitate binding and entry or the virus into the cell. Another class of antiviral drugs, which are effective with HIV, act by interfering with the synthesis and assembly of the coat proteins into the viral capsid. The US pharmaceutical industry already has the capacity to produce millions of doses of small molecules, so the rate limiting step in this case is more likely to be at the laboratory research and development stage.
Needed Public Investments
The initial Congressional vote for $8.3 billion to speed up the response to the coronavirus was a step in the right direction. The March 27 CARES Act stimulus vote went further on the biomedical research front, providing $1 billion to the NIH, $4.3 billion to the CDC, and $ 3.5 billion to the Biomedical Advanced Research Projects Authority. This is still far from what is needed to address the public health need.
Covid-19 and other emerging infectious diseases represent global threats to our national security. They require a substantial increased investment in fundamental biomedical science, if we are to develop the tests, vaccines, and remedies required to protect those at risk in a reasonable timeframe. Just as we have massive and sustained funding for the military and other efforts to fight more traditional and visible threats to our national security, the “invisible” killers require a similar budgetary effort.
The system already lacked sufficient funds to continue with vaccine development for the SARS-CoV virus after that threat subsided. We need a scientific and biomedical research infrastructure that can respond to the next threats, and of course a healthcare system and healthcare financing that can ensure high quality treatment for all.
Resources:
Cornonavirus Structure
American Public Health Association
Inactivation
Vaccine Development
Review of Treatments and Vaccines
Jonathan King and Eric Sundberg have directed biomedical research projects on viruses and viral proteins supported by the National Institutes of Health and National Science Foundation. They are both members of the Public Affairs Committee of the Biophysical Society. Melissa Kosinski-Collins has led HHMI and AAU-funded research programs in Biology Education.