I came to BPS 2020 with one intention, to search and learn about something which I don’t know already but is currently hot in the biophysics community. BPS attendees would agree that Cryo-EM was the winner by a landslide among any set of candidates. I took a dive and wrote this article for non-experts like myself who are interested in scientific developments currently happening in the field.
Before jumping into Cryo-EM, I found that it was appropriate to discuss a brief background and timeline of development of modern-day microscopy in order to put the importance of Cryo-EM into context.
Roughly around 1000 AD “reading stone” was invented. It was merely a spherical piece of glass which, if you kept on top of a text, would aid readability. About 300 years later Salvino D’Armate created first wearable eye glasses. Another 300 years later or so, Hans Martens/Zacharias Janssen claimed to have invented a microscope while experimenting with multiple lenses in a tube and in 1609 Galileo developed the first arrangement with a convex and a concave lens which is known as a compound microscope today.
This opened up a saga of trying to see the “unseen” and in 1665 Robert Hooke coined the word cell for the structure he observed in a cork bark and published Micrographia. The universe wasn’t the same anymore. Regular reports of discovering smaller and farther objects kept appearing until in 1860s Ernst Abbe published Abbe Sine Condition which gave a systematic way to find lens combinations and provided calculations for maximum possible resolution of microscope. I always used to wonder the why can’t we just keep on magnifying until we see the tiniest of objects? The answer is that the diffraction limit keeps us from “seeing” objects which were smaller than roughly half the wavelength of visible light as they can’t “interact” with visible light. And this limited our capacities to “observe” molecules. This limit was presented to us because visible light was being used as an interference source but then people started using X-rays and observe diffraction patterns of crystallized samples which allowed to penetrate us even further into the nano world. The glass-ceiling of diffraction limit was shattered when Ernst Ruska sped the electrons in a vacuum tube until their wavelength was really short. This made it possible to view the objects as small as the diameter of an Atom.
This was the birth of Electron Microscopy during the course of which several Nobel prizes were won, and millions of objects including structures of many organelles inside the cells were discovered which form the basis of the science as we know it today.
 But we have just reached 1931 so far. Several advances followed. In 1962, Green Fluorescent Protein (GFP) was discovered in the jellyfish Aequorea Victoria. GFP fluoresces bright green when exposed to blue light. GFP could be expressed inside the cells or attached to some organelles inside the cell and thus dynamics inside the cell can be observed by capturing the time-lapse of fluorescence. While GFP and similar probes give us more details about the insides of the cell, they still fail to give a holistic picture because of they are limited to observing a few types of organelles at a time. Parallel development of Confocal Microscopy and Total Internal Refraction Fluorescence Microscopy made it possible to perform imaging at a higher resolution and a better signal-to-noise ratio.
But we have just reached 1931 so far. Several advances followed. In 1962, Green Fluorescent Protein (GFP) was discovered in the jellyfish Aequorea Victoria. GFP fluoresces bright green when exposed to blue light. GFP could be expressed inside the cells or attached to some organelles inside the cell and thus dynamics inside the cell can be observed by capturing the time-lapse of fluorescence. While GFP and similar probes give us more details about the insides of the cell, they still fail to give a holistic picture because of they are limited to observing a few types of organelles at a time. Parallel development of Confocal Microscopy and Total Internal Refraction Fluorescence Microscopy made it possible to perform imaging at a higher resolution and a better signal-to-noise ratio.
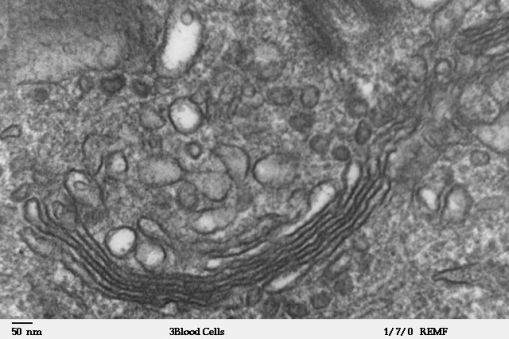 Atomic Force Microscopes (AFM), which uses a nanometer scale diameter cantilever, Tunneling Electron Microscope (TEM), and Scanning Electron Microscope (SEM), which use a beam of electron, were developed which scan the surface of a specimen, much like a blind person reading a text, and generate a topographical image of the sample. All of them won their inventors Nobel prizes. But some material, especially biomolecules, can be destroyed using these techniques. Around 1995, Stefan Hell pioneered Super Resolution Microscopy which allows us to “capture images” with higher resolution than was previously thought possible. He won Nobel prize in Chemistry in 2014. But super resolution microscopy doesn’t probe at an atomic level. All these advances were supplemented by developments in computational and detector technologies which I am not even getting into.
Atomic Force Microscopes (AFM), which uses a nanometer scale diameter cantilever, Tunneling Electron Microscope (TEM), and Scanning Electron Microscope (SEM), which use a beam of electron, were developed which scan the surface of a specimen, much like a blind person reading a text, and generate a topographical image of the sample. All of them won their inventors Nobel prizes. But some material, especially biomolecules, can be destroyed using these techniques. Around 1995, Stefan Hell pioneered Super Resolution Microscopy which allows us to “capture images” with higher resolution than was previously thought possible. He won Nobel prize in Chemistry in 2014. But super resolution microscopy doesn’t probe at an atomic level. All these advances were supplemented by developments in computational and detector technologies which I am not even getting into.
Even after so many developments, all these techniques lacked something which can be classified into two broad categories. Either they do not probe to an atomic level (even super resolution microscopy) or if they do, they tend to be invasive (like crystallization for x-ray diffraction) and/or can destroy some samples (like in SEMs, TEMs and AFMs). This is when Cryogenic Electron Microscopy (Cryo-EM) comes into picture.
In Cryo-EM, samples are plunged frozen to cryogenic temperatures and embedded in vitreous water. Then a softer electron beam is used to interact with the sample which does not destroy the sample or evaporate the water around it (unlike TEMs). This does not require crystallization either, unlike x-ray crystallography. This technique still does not allow you to observe the dynamics but gives a holistic snapshot of the sample in their native form with super refined details. Moreover, by slightly modifying the technique, you can tilt the sample and observe from multiple angles to get a 3D image of the sample. This technique is known as Electron Cryotomography (Cryo-ET).
![Cryo EM structure of Zika Virus [1] Cryo EM structure of Zika Virus [1]](/Portals/0/BPSAssets/Blog/Cryo%20EM%20structure%20of%20Zika%20Virus.jpg) These salient features make Cryo-EM attractive for studying a wide variety of samples. For example, one can visualize a complete picture of relative arrangements of the organelles inside the cells. In 2010, researchers at UCLA used Cryo-EM to visualize the atoms of a virus. Cryo-EM was named method of the year by Nature Methods in 2016 and the developers of the technique won Nobel Prize in Chemistry in 2017.
These salient features make Cryo-EM attractive for studying a wide variety of samples. For example, one can visualize a complete picture of relative arrangements of the organelles inside the cells. In 2010, researchers at UCLA used Cryo-EM to visualize the atoms of a virus. Cryo-EM was named method of the year by Nature Methods in 2016 and the developers of the technique won Nobel Prize in Chemistry in 2017.
BPS2020 saw a wide-ranging application of Cryo-EM to observe many more important biomolecules and infer their functioning. Searching Cryo-EM in BPS events application showed 130 abstract submitted and roughly 40 sessions which discussed Cryo-EM in some capacity. Some sessions discussed molecular mechanisms of GPCRs, other revealed the mechanical cycle diversity in kinesin superfamily (this happens to coincide with my own area of interest) using Cryo-EM. Many talks and posters discussed the signaling pathways inside and outside the cell as identified by Cryo-EM studies giving us indication about how information is transmitted across and inside the cells. All these findings are extremely important in themselves as they collectively enhance our understanding of mechanisms and working of this amazing entity called the cell which is the fundamental unit of life itself. This also readily provide information relevant to drug discovery for a number of diseases including but not limited to cardiac and neurodegenerative diseases. Cryo-EM has opened up channels for multipronged investigation aimed towards better understanding of biological sciences which is sure to lead to better diagnostics and healthcare related discoveries. It is certainly a very exciting time for cell biologists and science as a whole as Cryo-EM is helping us in redefining how we used to “see” things in the past.
References, sources and further readings:
[1] Sirohi, Devika et al. “The 3.8 Å resolution cryo-EM structure of Zika virus.” Science (New York, N.Y.) vol. 352,6284 (2016): 467-70. doi:10.1126/science.aaf5316
[2] Bellis, Mary. "History of Microscopes." ThoughtCo, Feb. 11, 2020, thoughtco.com/microscopes-timeline-1992147
[3] https://www.sciencelearn.org.nz/resources/502-types-of-electron-microscope
[4] https://en.wikipedia.org/wiki/Timeline_of_microscope_technology
[5] Green Fluorescent Jellyfish Photo by Willy Arisky from Pexels
[6] TEM image of white blood cell (taken from Science Learning Hub – Pokapū Akoranga Pūtaiao, University of Waikato, www.sciencelearn.org.nz)
Feel free to contact me on [email protected] for further discussion or inputs/comments about this article. I am always happy to discuss and share ideas about microscopy.