ROCKVILLE, MD – Many premature infants need mechanical ventilation to breathe. However, prolonged ventilation can lead to problems like respiratory diseases or ventilation-induced injury.
Jonas Naumann and Mareike Zink study the physics of mechanical stress from ventilation at Leipzig University, in Leipzig, Germany and discovered some of the mechanisms that explain why premature lungs are especially sensitive to stress. Naumann will present their research at the 68th Biophysical Society Annual Meeting, to be held February 10 - 14, 2024 in Philadelphia, Pennsylvania.
When you breathe normally, your diaphragm and the muscles between ribs create a negative pressure inside the lung. “But when you are undergoing mechanical ventilation, you are creating hydrostatic overpressure. And the forces which are acting during mechanical ventilation are completely different than during normal breathing. And this is probably causing some kind of damage to the cells,” Zink explained.
Using lung tissue from fetal and adult rats, the researchers together with collaborators from the Division of Neonatology, University Clinic Leipzig, used varying amounts of tension with rest phases in between, similar to the actions that occur within the lung during mechanical ventilation. Even with a little pressure, the premature rat lung tissue showed characteristics of being both elastic and viscous. This means the lung tissue changed its shape and responded to stress in a way that wasn't normal. Moreover, they found that “the fetal lung is much stiffer than the adult lung under deformation,” said Naumann.
To determine whether these tension-related changes in the tissue led to alterations in sodium transport, which is important for removing the water from the lungs that is present at birth, the team used electrophysiology to measure the movement of ions across a layer of premature lung cells. They found that changes in pressure affected the activity of two channels involved in sodium transport—the epithelial sodium channel and the sodium-potassium ion pump in the cells of lung alveoli. This disruption in the normal function of these transporters could explain why mechanical ventilation has negative effects on the infant's lungs.
“This may be the reason why lung fluid cannot get absorbed that well into the circulation after the preterm births,” Naumann explained. He hopes that there will be more research about what ventilator settings might lead to the best outcomes for preemies. Naumann points out that “small pressure gradients can have such a big impact on the lung mechanics.”
The next phase of their research will be exploring how the lung tissue’s extracellular matrix, the scaffolding and the glue that holds cells together, plays a role in mechanical ventilation. By better understanding how the premature lung responds to pressure, they hope that future studies improve therapies for babies born early.
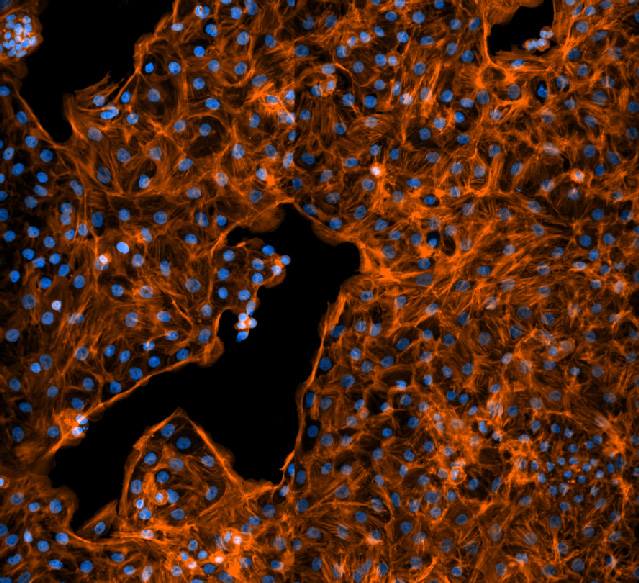
Rat fetal lung cells like those used to measure sodium transport. Cell nuclei are shown in blue and in orange are the actin filaments in the cytoskeleton. Image courtesy of Jonas Naumann.