With May being designated Arthritis Awareness Month by the Arthritis Foundation, BPS went searching for a member conducting research related to this debilitating condition. We were fortunate to find Alan Grodzinsky, Director of the Center for Biomedical Engineering at MIT, whose research group studies problems motivated by diseases of the musculoskeletal system including arthritis, connective tissue pathologies and, more generally, the molecular biology and biophysics of the extracellular matrix. Grodzinsky offers us a glimpse of the exciting work going on in his lab related to osteoarthritis.
What is the connection between your research and Arthritis?
We work on biophysical, biological and biochemical aspects of osteoarthritis (OA), a disease that has been estimated to affect over 150 million individuals worldwide. OA is a complex disease caused by a combination of mechanical and biological factors. It is not a single disease, but rather constitutes a heterogeneous combination of subtypes associated with risk factors for initiation and progression that include age, gender, genetic factors, obesity, improper mechanical joint articulation and congenital deformities of joints. Importantly, it is now recognized that OA is not just a disease of old people; traumatic joint injury in young active individuals involving knee ligament and meniscal tears, for example, can lead to OA at a young age. These injuries are especially common in young women of high school and college age and are known to progress to OA within 10‐15 years. Finally, OA is much more common than rheumatoid arthritis (RA), the latter involving autoimmune pathways distinct from the pathology of OA.
Why is your research important to those concerned about these diseases?
In our lab, we use in vitro models of joint injury involving living cartilage specimens from animal or human donor sources. Cartilage subjected to mechanical injury is co‐cultured with inflammatory cytokines known to be present in human joint synovial fluid during the weeks following a traumatic joint injury. We use these in vitro systems to try to quantify the cell biological/biochemical pathways of cartilage degradation that is a hallmark of progression to OA, pathways that are additionally initiated by mechanical damage to cartilage that can be imposed in a quantitative fashion using incubator‐housed loading instruments. These instruments also enable us to measure the biophysical changes in cartilage material properties to can occur in vivo. Additionally, these in vitro systems provide invaluable means to study the efficacy of potential drugs that can halt cartilage degradation and progression to OA. We have an active program for the design of nanoparticles that can be functionalized with small molecule or biologic drugs; the nanoparticles are designed to home directly into cartilage (and other adjacent soft tissues) for drug release inside cartilage after intra‐articular injection, thereby avoiding problematic systemic side effects that may be associated with sustained release of many otherwise suitable drug candidates. Finally, we also have a research program in our group aimed at the repair of cartilage defects which, if not otherwise treated, could rapidly lead to OA and joint failure. These studies involve the use of a functionalized self‐assembling peptide hydrogel scaffold that will soon be incorporated into animal studies in vivo.
How did you get into this area of research?
As an electrical engineer by training, I spent a year early on in my career on sabbatical at Children’s Hospital in Boston, working with Dr. Mel Glimcher, who was then Chief of Orthopaedic Surgery and a leading basic scientist in bone and cartilage research. Since cartilage is the mostly highly electrically charged tissue in the body (due to the presence of negatively charged proteoglycans (aggrecans) in the tissue matrix), I found that my deep interest in this subject could fit right into an academic career in research and tissue at the interface between engineering and biology. Continuous ongoing collaborations with cell biologists, extracellular matrix biochemists and clinicians have been extraordinarily helpful and exciting.
How long have you been working on it?
There is still no “cure” for OA, and there are currently no disease modifying drugs available (unlike the situation with RA, where new biologic drugs have emerged during the pasts 15 years that help ~65% of the individuals afflicted with RA). As a result, we and many other groups around the world are still working actively in many aspects of this field to try to achieve a better understanding of ways of halting disease progression, regenerating injured cartilage tissue and eventually identifying drugs that can halt the progression of OA disease. My research group has been involved in various evolving aspects of this research since the late 1970s.
Do you receive federal funding for this work?
Most of our funding related to cartilage biophysics, OA disease, and cartilage repair has comes from NIH. We’ve also received important funding over the years from programs within NSF, especially targeted to the discovery and use of biophysical tools for studies of cartilage and matrix molecular nanomechanics.
Have you had any surprise findings thus far?
Researchers in our group have recently discovered pathways and potential therapeutics that may help to preserve the collagen network of cartilage even after initial loss of other essential matrix components (such as aggrecan). However, delivery of such therapeutics to involved tissues, like cartilage, has not been solved. But students in our group have discovered that highly positively charged nanoparticles may provide an ideal means to home into cartilage with attached drug molecules in tow. So we’re pursuing those studies and also using the same potential therapeutics functionalized to hydrogel scaffolds for cartilage repair. In addition, Atomic Force Microscopy‐based imaging of cartilage tissue molecules and nanomechanical properties of cells and matrix have opened a window to the study of cartilage repair that we did not anticipate at all.
What is particularly interesting about the work from the perspective of other researchers?
The interdisciplinary nature of this kind research has been one of the most important and exciting features that have been of great interest to a wide variety of researchers in the field.
What is particularly interesting about the work from the perspective of the public?
Of course the hope of this kind of research is that it may lead to advances in diagnostics and patient treatment for OA disease, which has been a major concern of the public for many decades. While OA is not necessarily life‐threatening, it is the major concern for our aging population in terms of quality of life and freedom from pain and disability that is so common as a result of OA.
Do you have a cool image you want to share with the blog post related to this research?
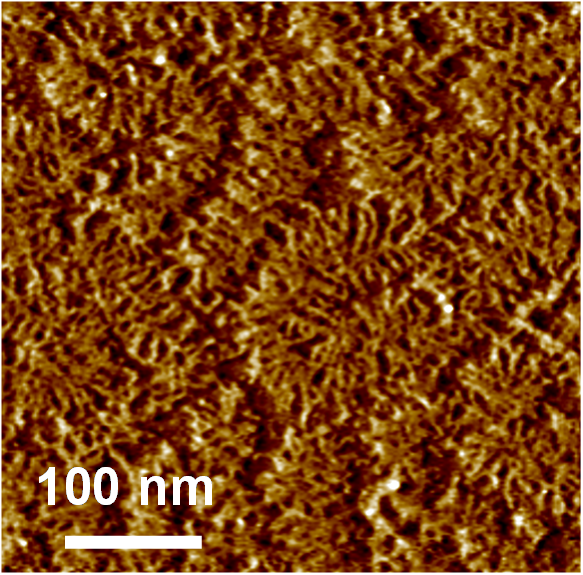 These are “aggrecan” macromolecules (tapping mode AFM imaging, Laurel Ng et al., J Structural Biology, 2003) which are critically important for the ability of cartilage in our joints to resist static and dynamic loading in our daily activities. These molecules are extremely densely packed inside cartilage and are the first molecules to be degraded and lost from cartilage at the very earliest stages of osteoarthritis. These molecules also regulate the transport of drugs to cells inside dense cartilage tissue, and they are currently the subject of nanomechanical and biophysical studies within our lab as a means to understand the progression of OA and attempts to stop OA.
These are “aggrecan” macromolecules (tapping mode AFM imaging, Laurel Ng et al., J Structural Biology, 2003) which are critically important for the ability of cartilage in our joints to resist static and dynamic loading in our daily activities. These molecules are extremely densely packed inside cartilage and are the first molecules to be degraded and lost from cartilage at the very earliest stages of osteoarthritis. These molecules also regulate the transport of drugs to cells inside dense cartilage tissue, and they are currently the subject of nanomechanical and biophysical studies within our lab as a means to understand the progression of OA and attempts to stop OA.