The Membrane Transport subgroup welcomes all members of BPS to join our symposium on Saturday afternoon, February 15, 2020, in San Diego. Our speaker line-up includes Osamu Nureki (University of Tokyo), Grace Brannigan (Rutgers University), Randy Stockbridge (University of Michigan), Katherine Henzler-Wildman (University of Wisconsin, Madison), and Jose Faraldo-Gomez (NIH). Please show your support by attending the symposium and registering for the subgroup!
Here, we highlight exciting research by two of our upcoming speakers, Randy Stockbridge (Fig. 1) and Grace Brannigan (Fig. 2), and one of our subgroup members, Luis Cuello (Fig. 3).
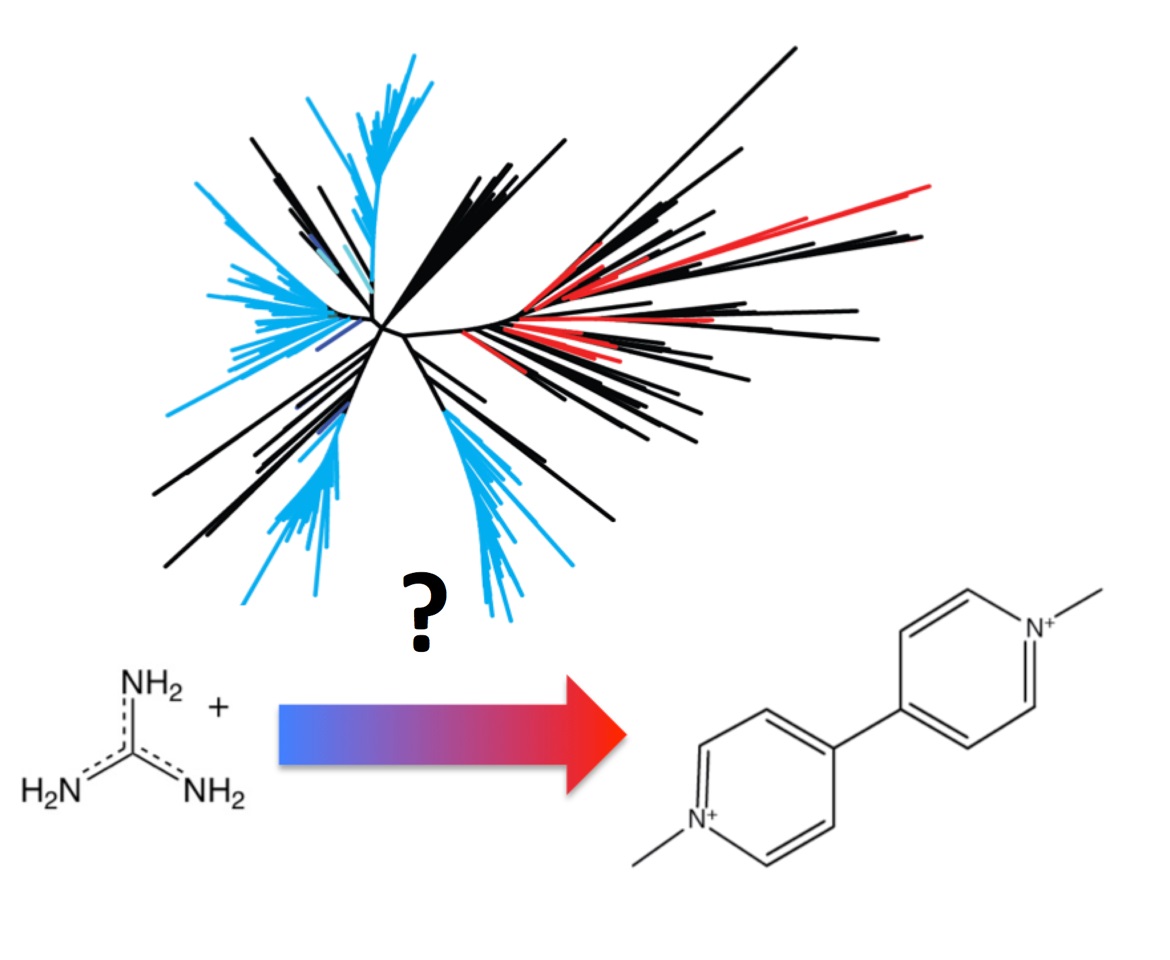
Work from the Stockbridge group (Fig. 1) is significant because the small multidrug resistance (SMR family) was named for its founding member, a promiscuous exporter of antibiotics and antiseptics, and has become an important model system in membrane protein mechanism and evolution. But most members of this family do not share drug export activity, and their function has been enigmatic. Here we show that the SMR family is mostly comprised of proteins that export guanidinium ion, a small, cationic metabolic byproduct. In addition to opening up an unusual bacterial physiology for further exploration, this work provides a comprehensive view of the SMR family and provides critical context to understand the evolution and mechanism of the multidrug transporters.
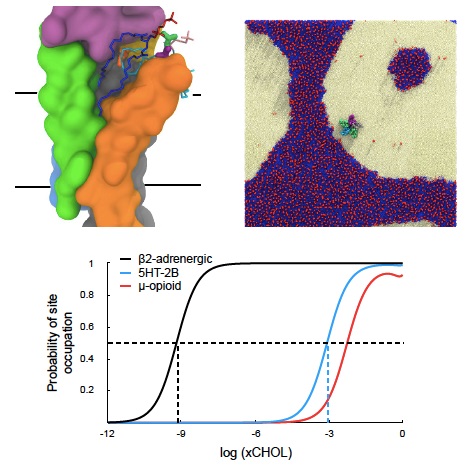
Work from the Brannigan group (Fig. 2) is significant because the function of ion channels can be particularly sensitive to lipid composition of the membrane. Most ion channels are centrally arranged oligomers, creating pockets at the subunit interfaces. Lipids interact with these and other pockets like ligands binding to receptors, but the membrane environment breaks many assumptions underlying classic ligand binding models. We have developed a general ligand-binding framework that can be applied to the binding of membrane-bound lipids, drugs, or hormones for transmembrane proteins. This approach holds particular promise for connecting experimental and computational results, distinguishing specific protein-lipid interactions from protein-membrane interactions, and identifying boundary lipids in ion channel structures.
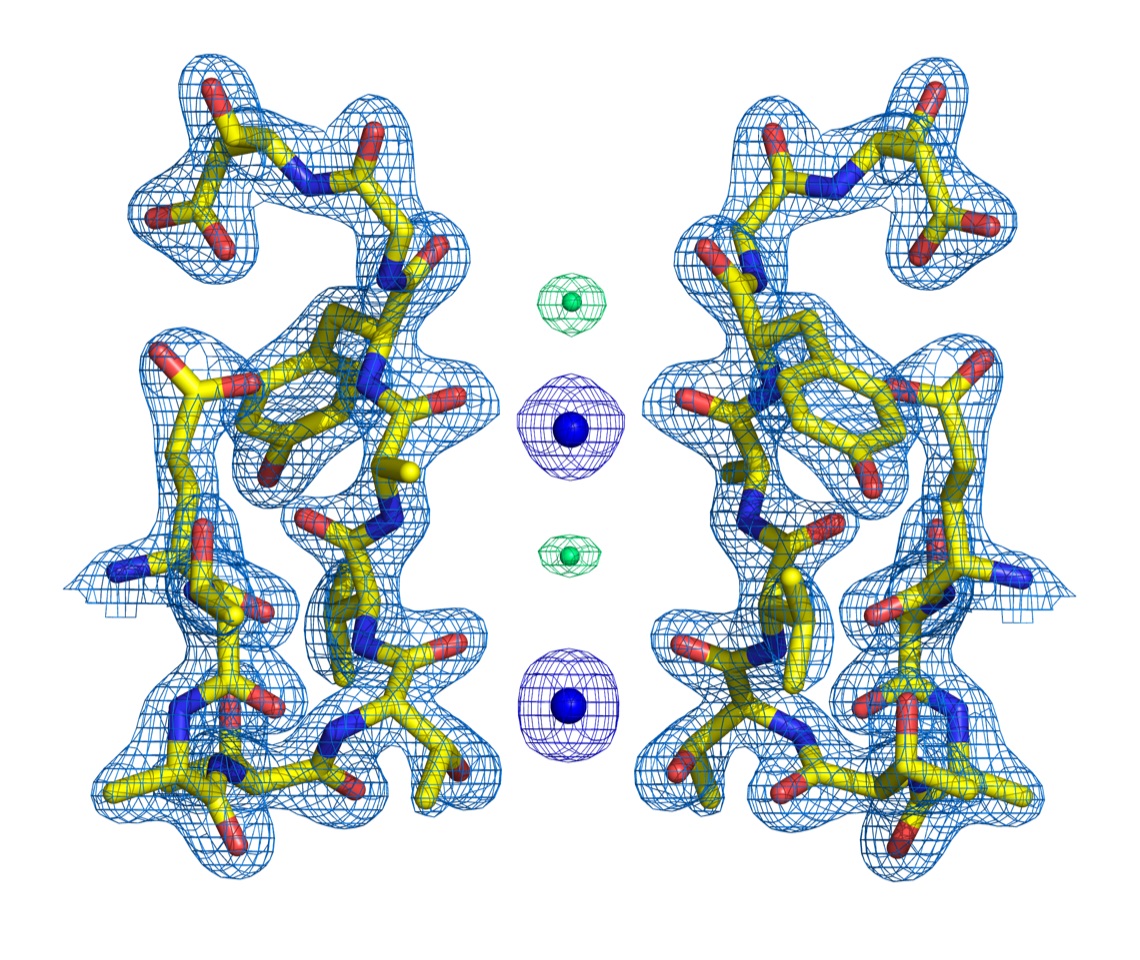
Work from the Cuello group (Fig. 3) is significant because crystal structures of K+ channels display 4 K+ ions bound to the selectivity filter. Two mechanistic models have been proposed to explain this experimental observation. The “canonical model” proposes 2 alternating ion-bound configurations (1,3 and 2,4) coexisting within a channel’s filter. Alternatively, in the “direct knock-on model” all binding sites are occupied at any given time, and ions establish direct contact. Here, it is shown the structure of a K+-channel selectivity filter stabilized in the 2,4-ion configuration, which provides a definitive experimental demonstration for the canonical model of ion permeation in K+ channels. In these structures, ions are separated by water molecules that seem to be co-transported during each ion transport cycle, which is in agreement with streaming potential measurements.
Stay tuned for more research highlights and more news about our Saturday subgroup meeting!
--Susan Rempe (Chair), Ming Zhou (Vice Chair), Lucie Delemotte (Secretary/Treasurer)