December 1 is World AIDS Day. Globally, there are an estimated 36.7 million people who have the virus. Despite the virus only being identified in 1984, more than 35 million people have died of HIV or AIDS, making it one of the most destructive pandemics in history.
In recognition of World AIDS Day, we spoke with Biophysical Society member Leor Weinberger, University of California, San Francisco, whose research focuses on how HIV makes a fate decision between active replication and latency.
What is the connection between your research and HIV/AIDS?
Our lab focuses on fundamental aspects of how HIV makes a fate decision between active replication (turning on) and a long-lived dormant state called latency (turning off). Latency is the chief barrier to curing a patient of HIV. We use quantitative single-cell approaches and mathematical models to map the transcriptional circuitry that controls this fate decision.
Many years ago we found that HIV harnesses and amplifies stochastic fluctuations (noise) in gene expression to control its active vs. latent fate decision (back in the early 2000’s HIV, in fact, provided the first experimental evidence of a noise-driven fate decisions). Recently, we discovered that noise can be manipulated with small molecules and we are developing therapies that disrupt HIV circuitry by manipulating noise.
Why is your research important to those concerned about HIV/AIDS?
HIV latency is the chief barrier to curing a patient of HIV. There is no vaccine for HIV and current anti-retroviral therapies only halt active viral replication; if a patient discontinues therapy, HIV will reactivate from latently infected cells and viral levels in the blood rebound to pre-treatment levels within a few weeks. We now know the most problematic (i.e., longest-lived) latent reservoir exists in a type of white blood cell called a CD4+ T cell, the same cell type that HIV actively infects. HIV remains ‘silent’ and integrated as a “provirus” in the genome of these cells. So, the field is actively pursuing approaches to better understand and attack the proviral latent reservoir.
Our work showed that a major mechanism HIV uses to promote its silencing is harnessing stochastic fluctuations in a transcriptional feedback circuit and gene-expression noise is now acknowledged as a primary clinical barrier to reversing HIV latency and curing HIV. Recently, we identified molecules that modulate stochastic fluctuations and could be a new class of therapeutic candidates for reversing latency.
How did you get into this area of research and how long have you been working on it?
I’d always hoped to make a contribution to medicine but as a student I became enchanted with the beauty of nonlinear dynamics theory and the intellectual approach of physics. This eventually led me to train with a group of physicists who were developing mathematical models to explain HIV infection dynamics in patients. At the time many physicists were modeling biology but very few models were grounded by experiment (HIV was one of the very few). A problem, however, was convincing experimental collaborators to test the most interesting predictions. So, in grad school I decided to learn wet-lab molecular biology and eventually started my own experimental lab.
This combination of modeling and experiment helps the science move faster. Plus, I greatly enjoy training students and postdocs at the interface of these disciplines and translating basic discoveries into potential therapies is both fun and rewarding. So, my lesson to aspiring biophysicists is: try to find training opportunities that cross disciplinary interfaces (theory and experiment, basic and applied, etc…) it can be challenging at times but is also very rewarding.
Do you receive public funding for this work? If so, from what agency?
We received generous funding to develop a radical new class of ‘autonomous’ antiviral therapies from the Defense Advanced Research Projects Agency (DARPA) as well as the NIH, especially the NIH Director’s Common Fund. DARPA actually started a program called INTERfering and Co-Evolving Prevention and Therapy (INTERCEPT) to fund this work. The NIH Common Fund was what allowed us to start out with this research; it is a unique and important program that funds high-risk, high-reward research through the Director’s Pioneer and New Innovator Awards and has mechanisms to support young researchers setting off in bold directions.
Have you had any surprise findings thus far?
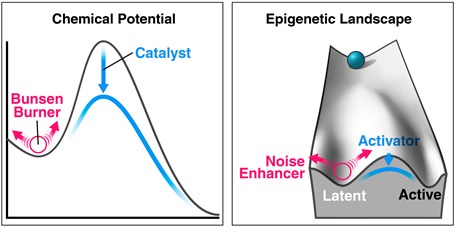
Probably our most surprising finding was discovering noise-enhancer molecules (Dar et al. Science 2014). These are small molecules (some are approved drugs) that alter transcriptional fluctuations without changing the mean level of gene expression and act like Bunsen burners for cells: they appear to potentiate cell-fate decisions in diverse systems.
The analogy can be made to physical and chemical systems, where many processes are enhanced by catalysts but also by increasing the thermal fluctuations (e.g., kT in the Arrhenius equation). Catalysts deterministically lower activation-energy barriers on potential-energy landscapes but when deterministic drivers are insufficient to cross the barrier, amplifying thermal fluctuations (e.g., with a Bunsen burner) provides an added perturbation for crossing activation-energy barriers.
Remarkably, we found that this concept applies to gene regulation during cell-fate decisions; the novel class of noise-enhancer molecules act like biological Bunsen burners. As a model system, we focused on HIV where the leading HIV-cure strategy requires latent virus be reactivated and then purged—but where current reactivation schemes are ineffective. Strikingly, these Bunsen burner-like noise-enhancer compounds potentiated transcriptional activators to greatly enhance HIV reactivation in patient cells. We also also identified noise-suppressor compounds (effectively ‘ice packs’) that inhibit reactivation.
It is important to note that noise modulators starkly contrast with generic stress responses (e.g., starvation), which can increase noise but necessarily attenuatetranscriptional activators. Thus, noise modulation is a fundamentally departure from generic stress responses. Preliminary evidence indicates that noise-modulating molecules could provide a general tool to manipulate diverse cell-fate decisions from antibiotic persistence to cellular reprogramming and cancer.
What is particularly interesting about the work from the perspective of other researchers?
I often hear from colleagues that they most appreciate that our work shows a biological role for stochastic ‘noise’. Before the HIV example, there had been beautiful and elegant work from colleagues measuring and identifying the sources of stochastic gene-expression noise in laboratory organisms (E coli and yeast). However, it was not clear if cells ‘cared’ about the noise or simply ignored it. The HIV example (Weinberger et al. Cell, 2005) was the first to experimental evidence that stochastic noise in gene-expression could flip a genetic switch and drive a biological fate decision (i.e., developmental bet-hedging). Developmental bet-hedging–the concept that organisms harness intrinsic variability to enable bet-hedging decisions between alternate developmental fates, similar to how financial houses diversify assets to minimize risk in volatile markets—had in fact been hypothesized since the 1960s. Subsequent work from some of my scientific heroes—and now friends—showed similar results in B subtillis, stem cells, and cancer cells. Recently, we have gotten a lot of requests from colleagues for our noise enhancer molecules and we send out samples every few weeks. I suspect that these molecules may end up being the more helpful contribution to the field.
What is particularly interesting about the work from the perspective of the public?
Typically, it is a different thread of research in our lab that catches the public’s attenuation. Many years ago we hypothesized the idea of treating infectious diseases by engineering therapies that co-evolve and co-evolve TIPs mentioned above (Metzger et al. PLoS Comp. Biol. 2011) has garnered the most public interest. Existing measures for infectious disease control face three ‘universal’ barriers: (i) Deployment (e.g. reaching the highest-risk, infectious ‘superspreaders’ who drive disease circulation); (ii) Pathogen persistence & behavioral barriers (e.g. adherence); (iii) Evolution (e.g. resistance and escape). To surmount these barriers, we have proposed a radical shift in therapeutic paradigm toward developing adaptive, dynamic therapies (Metzger et al. 2011). Building off data-driven epidemiological models, we show that engineered molecular parasites, designed to piggyback on HIV-1, could circumvent each barrier and dramatically lower HIV/AIDS in sub-Saharan Africa as compared to established interventions. These molecular parasites essentially steal replication and packaging resources from HIV within infected cells thereby generating Therapeutic Interfering Particles (TIPs), which deprive HIV of critical replication machinery thereby reducing viremia. The fundamental departure from conventional therapies is that TIPs are under strong evolutionary selection to maintain parasitism with HIV and will thus co-evolve with HIV, establishing a co-evolutionary ‘arms race’ (Rouzine and Weinberger, 2013). Like Oral Polio Vaccine, (OPV)—currently used for the W.H.O. worldwide polio-eradication effort—TIPs could also transmit between individuals, a recognized benefit for OPV. TIP transmission would occur along HIV-transmission routes (via identical risk factors), thereby overcoming behavioral issues and automatically reaching high-risk populations to limit HIV transmission even in resource-poor settings.