September is Prostate Cancer Awareness Month in North America. We spoke with Biophysical Society member Eleonora Zakharian, University of Illinois College of Medicine, about her research related to prostate cancer.
Prostate cancer (PC) is one of leading threats to men’s health and the second cancer-related cause of death in North America, with 220,800 new cases and about 27,540 deaths occurring annually1. The incidence of PC increases with aging. At early stages, PC cells depend on androgen for growth and survival, and androgen-ablation therapy has been the mainstay in early PC treatment and tumor regression. However, numerous retrospective studies have reported cases of cancer recurrences with anti-androgen therapy and relapse with an aggressive phenotype of androgen-independent PC. Further, treatments at the late androgen-refractive stage of PC are relatively inefficient2. The main features of late stage PC are an increased cell proliferation and apoptosis resistance.
What is the connection between your research and prostate cancer?
The path that connected our research to PC investigation has been drawn by an ion channel TRPM8. The TRPM8 protein has been the major focus area in my laboratory for several years. TRPM8 serves a role of a cold and pain receptor in the peripheral nervous system. However, the protein is highly expressed in the prostate, and its mRNA is upregulated in prostate cancer. In the recent years, we targeted to answer the question on the role of TRPM8 in the prostate and PC. And here is what we found:
The Melastatin group Transient Receptor Potential member TRPM8 is a Ca2+-permeable cation channel involved in cold and pain perception in the somatosensory nervous system3,4. The role of TRPM8 in thermosensation and its potentiation by chemical compounds such as menthol, eucalyptol, icilin that elicit cooling sensation are now well recognized and widely applicable in therapeutic treatments of acute pain. However, a fundamental gap existed how the channel is regulated endogenously? Indeed, the abundant TRPM8 expression, that was first documented, originated not from sensory neurons but the prostate epithelium5. But then the question on “what is the purpose of having a cold receptor that is activated by temperature below 28°C in the prostate gland” was lingering in the field since 2002. Approaching this question, we had a clear vision and understanding the necessity of identifying endogenous agonist for the channel. Serendipitously, our first choice fell on testing the effect of androgens on the TRPM8 action, and it happened to be the right one. Recently, we presented appealing evidence comprising of biochemical and biophysical studies that testosterone is a highly potent and specific agonist of TRPM8 channels6,7.
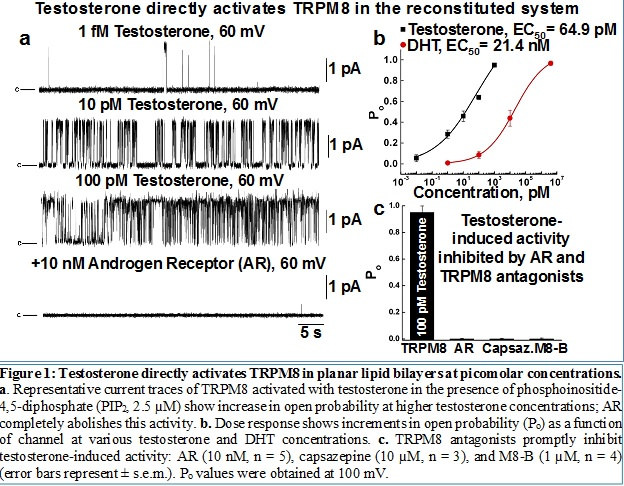
Why is your research important to those concerned about prostate cancer?
The next question that we approached was what is the role of the novel testosterone receptor TRPM8 in the prostate and prostate cancer. Although, TRPM8 mRNA levels are upregulated during PC progression, this upregulation is not adequately translated to the TRPM8 protein levels. Our recent critical discovery justified that TRPM8 is aggressively targeted for degradation in PC cells. Recovery of the protein effectively suppressed tumor cells growth. Thus, we identified that TRPM8 is a key element of the orphan pathway of non-genomic testosterone-induced responses, and its activity significantly contributes to anti-tumor defense mechanism.
How did you get into this area of research?
Initially, we have been focusing on TRPM8 channel function/structure relationship, and identified a number of unusual post-translational modifications on the channel that strongly impact its function 8,9. We also explicitly characterized the TRPM8 channel in the reconstituted system, using planar lipid bilayers approach 8,10. This system allowed us to demonstrate that TRPM8 is an intrinsic cold receptor. After the role of TRPM8 in cold perception and nociception was established, we proceeded towards identification of its role in the prostate, which later opened new horizons for defining designation of TRPM8 as a testosterone receptor 1,6.
How long have you been working on it?
We have been focusing on TRPM8 since 2005, and on the role of TRPM8 in the prostate cancer since 2012.
Do you receive public funding for this work? If so, from what agency?
Our initial work on TRPM8 has been funded by the National Institute of General Medical Sciences (NIMGS, R01GM098052).
Have you had any surprise findings thus far?
Our finding of TRPM8 as a testosterone receptor was very surprising to us.
Interestingly, however, the success of this discovery derived not from the conventional Ca2+ imaging or patch clamp experiments, but from using defined reconstituted system, which is well established in our lab. Testing the effect of androgens on TRPM8, initially we screened the channel responses in the heterologous system, using Human Embryonic Kidney (HEK) 293 cells stably expressing the protein. We found no dramatic Ca2+ uptake in response to testosterone, but unexpectedly an inhibition of menthol-induced responses upon sequential applications. The interpretation of this inhibitory testosterone effect seemingly suggested rather an antagonistic action of the steroid. To further confirm that testosterone antagonizes TRPM8, we evaluated its effect in planar lipid bilayers. To our great surprise, testosterone effectively activated TRPM8 in the bilayers, which revealed its true agonistic action, but then what went wrong using HEK-293 cells. Only months later we realized that culturing the cells in the presence of serum saturates the channel with testosterone, which causes its desensitization to the steroid. Depriving the steroid, or growing the cells in serum-free media solved this mystery7. Together, this story demonstrated remarkable advances for evaluating protein activity in planar bilayers, which we ordinarily perform and what made this discovery possible.
The next question was how is then TRPM8 implicated into the hormonal signaling of the prevalent male hormone, and how could it escape attention serving a role of a receptor for such a popular sex hormone? The realistic answer to that question was even greater of a surprise: the immediate testosterone receptor has never been identified before. It is paradoxical that although testosterone is known to affect a vast number of physiological processes, its direct contribution was mainly unknown. Indeed, the most recognized testosterone role in anabolic processes is mediated by its reduced analog dihydrotestosterone (DHT), to which testosterone is converted by 5-α-reductase when enters the cell. It is actually DHT that binds the classic androgen receptor (AR) and stimulates protein synthesis by transactivation of nuclear genes. Alternatively, testosterone is a direct precursor of aromatase-mediated conversion to estrogen, which then activates the estrogen receptor (ER) and its subsequent targets. Remarkably, TRPM8 and the androgen receptor have opposite preferences for binding testosterone and DHT. More precisely, DHT has nearly 10-fold higher AR affinity than testosterone11, while testosterone has almost 1000-fold higher TRPM8 affinity than DHT 7. Finally, TRPM8 presence on the plasma membrane initiates direct contact with the steroid, even before it enters the cell for further conversion to DHT. Hence, the TRPM8 protein appears to be the first immediate receptor for testosterone with all its subsequent physiological implications.
What is particularly interesting about the work from the perspective of other researchers?
Our findings foreordained new directions for both the TRPM8 protein and the steroid. After more than a decade of intensive studies on TRPM8 as a cold receptor and nociceptor in the peripheral nervous system, the channel function has been well elucidated: starting from native and heterologous systems, justifying its role in nociception12, and adjourning its intrinsic cold sensitivity in the reconstituted system10. Except for, perhaps, its unresolved structure, which yet remains quite challenging as TRPM8 is heavily modified at the post-translational level8,9. With our recent contribution, eventual identification of the purpose for the abundant TRPM8 expression in the prostate in its novel role as a steroid receptor opened new horizons for its physiological designation, and it may take another decade to unravel and characterize this system.
What is particularly interesting about the work from the perspective of the public?
The importance of our work could further have a significant impact on the development of a novel therapeutic strategy to fight prostate cancer. Indeed, we showed that TRPM8 is a tumor suppressor that is destroyed as prostate cancer progresses. Recovery of TRPM8 may, therefore, be applicable for a novel therapeutic intervention.
References
1 Jemal, A. et al. Cancer statistics, 2009. CA: a cancer journal for clinicians 59, 225-249, doi:10.3322/caac.20006 (2009).
2 Martel, C. L., Gumerlock, P. H., Meyers, F. J. & Lara, P. N. Current strategies in the management of hormone refractory prostate cancer. Cancer Treat Rev 29, 171-187 (2003).
3 McKemy, D. D., Neuhausser, W. M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52-58, doi:10.1038/nature719 (2002).
4 Peier, A. M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705-715 (2002).
5 Tsavaler, L., Shapero, M. H., Morkowski, S. & Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer research 61, 3760-3769 (2001).
6 Asuthkar, S. et al. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. The Journal of biological chemistry 290, 2659-2669, doi:10.1074/jbc.M114.610824 (2015).
7 Asuthkar, S. et al. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. The Journal of biological chemistry 290, 2670-2688, doi:10.1074/jbc.M114.610873 (2015).
8 Zakharian, E., Thyagarajan, B., French, R. J., Pavlov, E. & Rohacs, T. Inorganic polyphosphate modulates TRPM8 channels. PloS one 4, e5404, doi:10.1371/journal.pone.0005404 (2009).
9 Cao, C. et al. Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function. Cell reports 4, 302-315, doi:10.1016/j.celrep.2013.06.022 (2013).
10 Zakharian, E., Cao, C. & Rohacs, T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 12526-12534, doi:10.1523/JNEUROSCI.3189-10.2010 (2010).
11 Litvinov, I. V., De Marzo, A. M. & Isaacs, J. T. Is the Achilles' heel for prostate cancer therapy a gain of function in androgen receptor signaling? The Journal of clinical endocrinology and metabolism 88, 2972-2982, doi:10.1210/jc.2002-022038 (2003).
12 Julius, D. TRP channels and pain. Annual review of cell and developmental biology 29, 355-384, doi:10.1146/annurev-cellbio-101011-155833 (2013).