 Blood clotting, thrombosis, and blood cells all have great biological and clinical significance. Clotting is necessary to stop bleeding yet thrombi can obstruct blood flow, which can cause heart attacks, strokes, venous thrombosis, and pulmonary embolism. Although much is known about various aspects of clotting, much less is known about clot contraction or retraction. Clot contraction is thought to play a role in hemostasis, wound healing and the restoration of flow past otherwise obstructive thrombi.
Blood clotting, thrombosis, and blood cells all have great biological and clinical significance. Clotting is necessary to stop bleeding yet thrombi can obstruct blood flow, which can cause heart attacks, strokes, venous thrombosis, and pulmonary embolism. Although much is known about various aspects of clotting, much less is known about clot contraction or retraction. Clot contraction is thought to play a role in hemostasis, wound healing and the restoration of flow past otherwise obstructive thrombi.
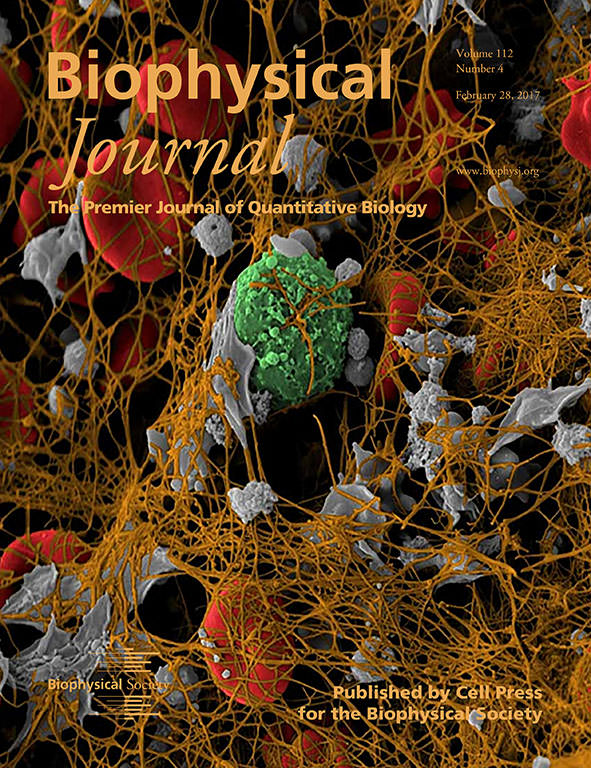
The cover image for the February 28 issue of the Biophysical Journal shows a colorized scanning electron microscope image of a coronary artery thrombus extracted from a heart attack patient. We chose this image because contraction occurs in such thrombi and all of the elements described in our paper are visualized here: platelets (gray), fibrin (brown) and red blood cells (red). Thus, this image represents a real-world example of the practical significance of our research. Furthermore, we have found that clot contraction is altered in patients with certain thrombotic disorders, such as acute ischemic stroke. Our model provides the fundamental mechanical basis for understanding the contraction of blood clots.
The contraction of blood clots and thrombi is an interdisciplinary problem related to fundamental aspects of cell biology, including cell motility and interaction of cells with an extracellular matrix. The biophysical mechanisms of clot contraction have been poorly understood, although it has been shown that it results from the interaction of actively contracting platelets with the fibrin network, the structural matrix of the clot that has unique mechanical properties. Though many of the same basic principles of motility of other cells are employed in this system, the specialized mechanisms of cellular contractility represent a novel biological application. The consequences of cell-matrix interactions in blood clots are unique and result in massive compaction of the network, rather than motility or alignment of fibers that occur in other cellular contractile environments.
Blood clot contraction is driven by platelet-generated contractile forces that are propagated by the fibrin network and result in clot shrinkage and deformation of red blood cells. We developed a model that combines an active contractile motor element with passive viscoelastic elements consisting of fibrin and red blood cells. This model predicts how clot contraction occurs due to active contractile platelets interacting with a viscoelastic material, and explains the observed dynamics of clot size, ultrastructure, and measured forces.
- Andre E.X. Brown, Chandrasekaran Nagaswami, Valerie Tutwiler, Hailong Wang, Rustem Litvinov, Vivek Shenoy, and John Weisel
Note: This image originally appeared in a different form in Science 325:651, 2009.